Can You Get Condensation on a Sponge?

Last week I taught a class called What the Duct!? at the Builders’ Boot Camp in Virginia. Paul Francisco was one of the other instructors (teaching about indoor air quality), and on the last evening at dinner, our conversation turned to building science. (Imagine that!) I don’t recall exactly how we got there, but somehow the question of condensation came up. Paul talked how Bill Rose, author of Water in Buildings, often asks the question, Can you get condensation on a sponge?
If you’ve read Rose’s book, you probably already know the answer. Well, let me rephrase: If you’ve read and absorbed the material in Water in Buildings, you know the answer. If you haven’t read the book, you may now be able to guess where I’m going with this. (But you should still get yourself a copy and read it anyway.)
On page 81 of the book, Rose quotes Leonard Haeger from the 1952 Condensation Conference: “I suppose in the beginning we should have a definition of condensation, and to practical men condensation is what you find on a highball glass at 5:30 in the afternoon.”
It’s also what you get on single pane windows (shown below), bathroom mirrors, and grass in the morning. It happens when air with a certain dew point temperature finds a surface that’s at or below that temperature. It occurs under precise conditions of temperature and water vapor content of the air. It’s a point on the psychrometric chart.

Sponges, of course, can have a specific temperature and interact with the water vapor in air. Unlike a glass surface, however, a sponge collects water from the air at a wide range of temperatures. It’s a sorptive material. It adsorbs and absorbs water on the surfaces and in the spaces of its pores.
Adsorption, briefly, refers to water that’s close to and bonded strongly to a surface. When a material pulls in so much water that some of the water molecules are in the pores but not bonded strongly to the surface, those water molecules are absorbed. The two phenomena together are referred to as sorption.
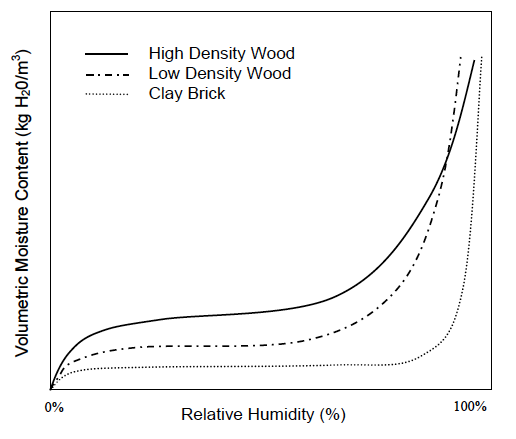
Wood and brick are like sponges in that regard. The graph below, called a sorption isotherm, is from the doctoral dissertation of Professor Chris Timusk. As you can see, the moisture content of the material depends on the relative humidity of the air. Even if the material temperature is higher than the dew point of the surrounding air, it can collect water. And the higher the RH, the more water it collects.
That’s not at all like condensation. As Rose says in his book, “Sorption is a better term, covering adsorption and absorption.” When you see condensation on the bathroom mirror, you know the dew point of the water vapor in the air is equal to or higher than the surface temperature of the mirror.
When you see a sponge that feels damp even though it hasn’t been used in a while, its temperature may or may not be below the dew point. But as a sorptive material, it doesn’t have to be. The higher the relative humidity, the more moisture it adsorbs and absorbs.
This issue comes up all the time in building science discussions, especially regarding what happens in exterior sheathing. I’ve been guilty of this, too. When it’s cold outside and a building doesn’t have exterior insulation, we often talk about “condensation” occurring when humid air from indoors finds its way to the interior side of the sheathing. As with the sponge, though, the moisture content of that sheathing depends on relative humidity, not dew point.
So, back to the original question: Can you get condensation on a sponge? When Paul Francisco brought this up at dinner last week, he told the story of being in a session where Bill Rose asked the question and then said no, you cannot get condensation on a sponge. He gave the reasons above to support that answer.
Paul then disputed Rose’s argument, saying yes, you can get condensation on a sponge. After you take a shower and have condensation on the mirror, you wipe it off with a sponge. That’s how you get condensation on a sponge.
Related Articles
Dew Point — A More Meaningful Measure of Humidity?
Psychrometrics – Impenetrable Chart or Path to Understanding?
Buried Ducts Risk Condensation in Humid Climates
Why Is Fall a Good Time of Year for Window Condensation?
Photo of blue sponge by gosheshe from flickr.com, used under a Creative Commons license.
NOTE: Comments are moderated. Your comment will not appear below until approved.
This Post Has 17 Comments
Comments are closed.

So in high humidity
So in high humidity environments like in coastal areas where I live, sheathing is going to have a higher moisture content unrelated to its surface temperature, correct? I’m guessing there is not a way of controlling this? Is this moisture content high enough to facilitate bacteria growth?
Matt wrote: “Is this moisture
Matt wrote: “Is this moisture content high enough to facilitate bacteria growth?”
Increased sorption in humid climates definitely increases the likelihood of mold growth. Whether it’s ‘high enough’ depends. Lew Harriman gave an excellent presentation at the 2015 Hot Dry Climate Forum where he discussed mold in depth, including the physics behind surface mold growth. Here’s a link: http://bit.ly/2frLBMo
Open cell spray foam acts
Open cell spray foam acts like a sponge. Heck it IS a sponge, as we’ve written before. When you go closed cell you HELP to eliminate all the eccentricities of psychrometrics if you have a fully encapsulated chamber as we do. Of course any wood framing is still a significant thermal bridge for a stud framed house – and a sponge as Allison notes. Fiber cement is a sponge as well – as Dr. Joe so wonderfully elaborates in his “Mind the Gap” article.
Hello Charles.
Hello Charles.
There has been a new advancement in ocSPF. We have developed an open cell that does not absorb water. It actually has a water absorption coefficient lower than ccSPF. The application of this product is to be an upgrade to standard mineral wool. This is a fairly new concept but i would other wise agree with you.
What brand of open cell is
What brand of open cell is that?
I completely disagree with
I completely disagree with this one. Allison and Paul must have had too much wine before this discussion. Sorption occurs at the chemical/molecular level. Sponges work on capillary action or surface tension, which is a macroscopic effect. The pores are what is holding the water, which is why you can easily squeeze the water out of the sponge. You cannot squeeze water out of wood. I will bet that if you use sponge material to make an object with no pores, it won’t “absorb” any significant amount of water. Maybe there are some sponges that are hygroscopic or sorptive, but I haven’t seen any. If I leave a soaked sponge out in the open long enough, it clearly “dries” out. If I stuck that sponge in the freezer, and pulled it out later, I bet that water will condense on it until it warms up. Then it will evaporate again. It is not a sorption process, at least to any significant extent.
Roy, I completely disagree
Roy, I completely disagree with you. Paul and I could not have had too much wine because we were drinking beer. But I think I may be in agreement with you about sponges. When Paul told the story, he said Bill Rose used that as an example when he talked about condensation, so I assumed it must be right. I need to ask Bill for his version of the story, though. Maybe something got lost in the translation. Or maybe it depends on the type of sponge.
Then again, I don’t buy your argument about using “sponge material to make an object with no pores.” If you could use brick material to make an object with no pores, it probably wouldn’t be hygroscopic either.
Let me see what I can soak up in my research.
Allison, I thought that I
Allison, I thought that I always saw you and Paul drinking wine at ASHRAE meetings. Do you switch to beer when you are with the “working” people 😉
I think that the mechanisms of “hygroscopicity” and “capillary action” are quite different in terms of their driving forces and probably can occur simultaneously. I would guess that brick has both, but I don’t know. But I will still claim that water “condenses” on any surface that is colder than the dewpoint of the surrounding air. Hygroscopic materials will absorb water vapor at even lower surrounding humidities, but capillary action (things with pores) need direct contact with liquid water for them to “absorb” water and will eventually dry out if in contact with air that is not saturated.
That was probably Jason. I do
That was probably Jason. I do drink wine but I don’t recall having done so with Paul at an ASHRAE meeting. We were drinking beer in Virginia, though, because at a place named Fatback Soul Shack, I trust the quality of the beer a lot more than I trust the wine. Plus, I like good craft beer.
I’m glad Bill Rose caught wind of the discussion and has chimed in here now.
Yea, Jason and Bruce are the
Yea, Jason and Bruce are the wine drinkers at ASHRAE. I used to drink Pabst until the hipsters drove the price up, then I switched to Pearl until I moved from Texas to Oklahoma. Now I just drink whatever 3.2 beer is on sale here.
I think you are going down
I think you are going down the wrong track thinking in terms of relative humidity, which is really the psychrometrics of the air. In a permeable solid where water is bound up in the lattice structure, water is at a lower energy state: you have to squeeze it to get the water out. This measure, called water activity, or a_w is what you really want to be looking at, because beyond a_w of 0.76, mould growth happens.
In latex:
a_w = exp{(frac{b T_d}{c + T_d}-frac{b T_s}{c+T_s})}
Where T_d= dew point T, and T_s=substrate T, b & c are constants the vary with the T ranges
So to me it is far more important to watch surface temperatures, noting especially the thermal bridges, than to look at the RH of a space.
Tim, you’re right that water
Tim, you’re right that water activity is the relevant measure for understanding moisture content and mold growth. But that’s not the point I’m trying to make here. I was explaining Bill Rose’s reasoning for why the word “condensation” doesn’t apply in many of the building science cases where it’s used. Relative humidity and sorption isotherms are indeed the right track for that discussion. If Roy is correct, though, a sponge may not have been the best material to use to illustrate that.
Paul F. just came
Paul F. just came breathlessly into my office to show me the blogpost. Hey, thanks for the kind words. The book is out of print and costs way more than it’s worth on Amazon (even the Kindle version went up when the paper version went up–go figure).
I do the mirror and sponge routine when I present, and I always credit Paul for the wiping the mirror bit.
RoyC challenges the role of sorption, which he correctly notes occurs at the chemical/molecular level. Water binds to molecules like the molecules of cellulose, and clay, and gypsum. The dry sponge sitting on the counter is not entirely dry–it loses weight with oven-drying. If we pressed out all the pores, there would still would be a small amount of water hugging the surface.
In my book I noted the three classical phases of water, of pure water, then described another “phase” of bound water–dirty water. The classical phases use the textbook values of binding energy for water-to-water. The binding energies to hygroscopic materials vary all over the place.
Take care, y’all. Gotta check on my daughter in Santo Domingo. By the way, I hear all these weatherheads talking about “most violent storm ever recorded” and “highest rainfall ever recorded”, and none of them say that storms will all be milder after this. They will, won’t they?
Back to RoyC. You have a
Back to RoyC. You have a point. I don’t know what the sponge is made of. I’ve had natural sponges and plastic sponges. Is there a difference between air-dry and oven-dry? I don’t know because I haven’t done it. I suspect that for an organic sponge it’s yes, and for a plastic sponge it’s close to no. And capillarity accounts for almost all the water in a wet sponge as you state.
Capillarity depends on the molecular level where the water meets the capillary wall. There would be no capillarity if the contact angle is 90 degrees.
I try to reserve the term “condensation” for where it’s water-to-water, and avoid it with water-to-material. That’s a losing battle.
Bill,
Bill,
I am not sure what you mean about capillarity and a contact angle of 90 degrees. My understanding is that hydrophylic surfaces have high attraction of liquid water with contact angles between zero and 90, and hydrophobic surfaces have contact angles between 90 and 180 degrees. On the former, water tends to “wet” the surface, whereas on the latter the water tends to “bead” up. I am guessing that sponges have contact angles less than 90 degrees. But then again, perhaps the sponge is just trapping beads of water in hydrophobic pores?
But I see three different mechanisms for water being discussed here. Condensation is simply water vapor condensing to a liquid because its temperature is lowered to a value below its dewpoint, or correspondingly, the water vapor pressure is higher than the saturation water vapor pressure at that temperature. You don’t even need a surface for this to happen (rain?). Sorption occurs because of molecular attraction that results in water vapor being absorbed or adsorbed from the air onto a hygroscopic material at even lower vapor pressures than those associated with condensation. Capillary action is purely a liquid water (not vapor) and surface material interaction. Sponges can probably exhibit all three depending on the material and temperatures, but I think that capillary action is what makes a sponge useful for cleaning up spills or drying surfaces.
Since I’m in the merry
Since I’m in the merry company of sober intellectuals. Can I be pedantic and propose that water-water should be called coalescence, water-to-impermeable-material should be condensation, and water-to-permebale-material would be sorbtion. And water-to-sponge should simply be called ‘drunk’.
On that basis, RH is a
On that basis, RH is a measure of vapour in air, and a_w a measure of moisture in a permeable material. I avoid talking in terms of RH because most people outside of building science would think of that as one measurement in a space. But we all know that at the boundary with a substrate the RH is going to change with the substrate temperature, and that plasterboard is going to have more of less sorbtion depending if its backed against a thermal bridge or insulation, respectively.