Is That Really Condensation?

Look at that photo above. Is condensation really responsible for all that moisture you see? We use the term “condensation” a lot when talking about water vapor interacting with materials. But when it comes to porous materials like wood, concrete, and drywall, that’s not really what’s happening. Ready to explore this rabbit hole?
Condensation occurs with nonporous materials
There is some condensation in that photo above. It’s the water dripping off that PVC pipe. Condensation is what happens when a nonporous material drops below the dew point temperature of the air it’s in contact with.

On what kind of materials does condensation occur? Here are a few:
- glass shower door
- mirror (also glass)
- metal window frame
- plastic pipes
- foil jacket on ducts
- porous materials with nonporous coatings like wood with varnish
In cases of true condensation, the water vapor interacts only with the outer layer of the material. And the interaction depends on the temperature of that material. When the material is above the dew point temperature, the surface is dry. When the material drops below the dew point, water vapor molecules stop just bouncing off and start sticking.
Adsorption occurs in porous materials
In the lead photo above, the wood is saturated with water. It started getting wet even before the temperature of the wood dropped below the dew point temperature. Why? Because the water vapor in the air is not just outside the material. It’s also inside the material, floating through the pores and sticking to the pore walls. The relevant psychrometric quantity in this case is relative humidity, not dew point temperature.
![Sorption isotherms for three porous materials [Courtesy of Prof. Chris Timusk]](https://www.energyvanguard.com/wp-content/uploads/2023/09/sorption-isotherm-porous-materials-chris-timusk-thesis.png)
After a bit, each curve flattens out as the pore surfaces get their initial coverage with water molecules. And then the moisture content of each porous material begins to rise steeply when the relative humidity gets high. That’s when the pores start becoming full of water.
Adsorption vs. absorption
When I wrote the word “adsorption,” that wasn’t a typo. Adsorption (the subject of my doctoral dissertation) is when molecules stick to the surface. In the case of water vapor and porous materials, the water molecules stick to the pore walls, one layer at a time. Those layers have a special name, too: monolayers.
Eventually, though, the pores fill up. Then you have liquid water and the material is now absorbing water.
In my freshman chemistry class, I learned a great way to distinguish between adsorption and absorption. There was a cartoon in my textbook that illustrated this concept with a pie. When someone throws a pie in your face, you’ve adsorbed it. When you eat a pie, you’ve absorbed it.
Understanding sorption isotherms
Now, back to the sorption isotherms. That steep rise at high relative humidity doesn’t depend on the dew point temperature. Instead, it depends on how big the pores are, the shape and distribution of the pores, and the relative humidity. This rabbit hole is a deep one, but if you want to understand things like buckling floors, wet sheathing, and mold, you may want to jump in.

As you can see in the sorption isotherm graph above, each material has its own shape when you plot this graph. The way building scientists create sorption isotherm graphs is with carefully controlled experiments with equipment like you see above.
They put a sample in the chamber. It sits on a scale that measures the mass. They gradually increase the relative humidity inside the chamber while maintaining a constant temperature (iso = same, therm = temperature). The mass increases as the relative humidity increases. Then they plot the data.
Temperature variation
Now let’s talk about temperature. Each sorption isotherm is measured at a constant temperature. But what happens when we measure sorption isotherms for the same material at different temperatures? It’s what we expect would happen, right? As we lower the temperature, the material absorbs more water.
![Moisture content of wood at different temperatures and relative humidity [Source: Forest Products Laboratory, Research Note FPL-RN-0268]](https://www.energyvanguard.com/wp-content/uploads/2023/09/moisture-content-wood-vs-relative-humidity.jpg)
Down the rabbit hole
I discussed this topic in my book, but let’s take a quick look here, too. The water molecule, one oxygen and two hydrogens, is polar. The oxygen lacks two electrons from having a complete outer shell. The hydrogen has one electron in its outer shell and would like one more. So the oxygen shares one electron with each hydrogen by creating covalent bonds. That makes the oxygen side slightly negative, the hydrogen side slightly positive.
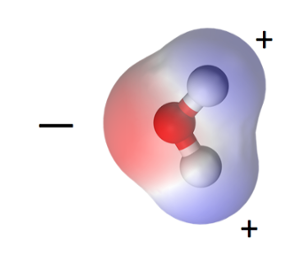
This polarity has enormous ramifications for how water interacts with materials. In fact, it has enormous implications for life on Earth. It’s this polarity that affects how it interacts with materials. That applies to what’s going on inside the pores of porous materials and on the surface of nonporous materials.
Want to go deeper? See chapter 6 of my book. Also, these two articles by Dr. Joseph Lstiburek can open up whole new pores in this rabbit hole:
It’s All Relative
Wood Is Good…But Strange
But the big takeaway here is that when we’re talking about porous materials, it’s not really condensation that’s happening. The term I like to use is moisture accumulation. And now that you’ve got this under your belt, you’re ready to join the building science nerds who argue about whether or not you can get condensation on a sponge.
Allison A. Bailes III, PhD is a speaker, writer, building science consultant, and the founder of Energy Vanguard in Decatur, Georgia. He has a doctorate in physics and is the author of a bestselling book on building science. He also writes the Energy Vanguard Blog. For more updates, you can subscribe to the Energy Vanguard newsletter and follow him on LinkedIn.
Related Articles
Can Cold Surfaces Wring Moisture From Dry Air?
Condensation on Storm Windows: A Lesson in Condensing Surfaces
3 Cool Videos about Hydrophilic, Hydrophobic, & Hygroscopic Materials
Can You Get Condensation on a Sponge?
Comments are welcome and moderated. Your comment will not appear below until approved.
This Post Has 10 Comments
Comments are closed.

Allison, There’s one effect of the polarity of water vapor that turns out to be very handy in daily life. If you apply a high frequency electromagnetic field to a batch of water molecules they will start to oscillate at the frequency of the E-M field. All that molecular jiggling causes a lot of internal friction and the molecule’s temperature will rise. Since virtually all foods contain water you can heat them in an E-M field because of the polarity of the water molecule with two hydrogens off to one side. And that’s what happens when you put food in a microwave oven! Thanks to that peculiar water molecule that is electrically neutral, but polarized by it’s molecular shape, illustrated in your sketch, it jiggles when excited by that E-M field. It would be interesting to read about many other effects of the polarity of water molecules that we are experiencing constantly and not aware of.
Gene: Yes, the microwave oven is a great example of how we take advantage of the water molecule’s polarity. Thanks for mentioning that here.
Van der Waals forces give water a number of unique qualities including the phenomenon of siphons. It’s kinda sticky to itself.
Have you seen this:
https://www.fpl.fs.usda.gov/documnts/fplrn/fplrn268.pdf
This gives what the moisture content is of wood exposed to the outdoors by location and month. It appears to be for building materials but thought it might apply to seasoning firewood as in the lowest possible drying content for firewood.
Any thoughts?
JayW: Thanks for sharing that link here. I’ve just added a table from that paper to the article.
In the graphs and tables above, is there an assumption that the wood and adjacent air are at the same temperature?
Roy: In the case of the graphs, the answer is yes. The sample sits in the middle of the chamber, and everything inside is at thermal equilibrium when they make measurements. I haven’t done those measurements myself, but that has to be the case if they’re measuring equilibrium moisture content. The table comes from a paper on wood being stored outdoors, but they’re still talking about equilibrium moisture content.
Allison, that’s what I thought, but then why do you say that the moisture content is a function of RH and not dewpoint? Since it is also a function of temperature, I could easily generate a table of moisture content as a function of temperature and dewpoint or any other humidity measurement and temperature.
Allison, I am also curious about this. The table implies that the rate of adsorption is dependent on both RH and temperature, not just RH. Am I missing something?
Great article! Thanks for sharing this. I’ve learned that vapor pressure is Pressure – capital P!